“Societal Challenges” pillar of Horizon 2020
This project gives an answer to the scope of the “Societal Challenges” pillar of Horizon 2020, under the “Health, demographic change and wellbeing” work programme and specifically under the “Better Health and Care, Economic Growth and Sustainable Health Systems” topic: SC1-BHC-15-2018: New anti-infective agents for prevention and/or treatment of neglected infectious diseases (NID).
Many diseases that are not life-threatening in developing countries do not receive the resources and attention required to investigate control, prevention or treatment. Cutaneous leishmaniasis (CL) is one of the most neglected diseases amongst the neglected tropical diseases (NTD).
Infections are common in low-income populations of developing regions. Producing (sometimes up to 200) ulcers on exposed parts of the body, CL can leave permanent scars that are often the source of major stigma, social prejudice and psychological damage and can have an economic impact.
Existing therapies have several drawbacks
- a variable efficacy
- safety issues
- stability problems
- high cost.
Moreover the current drugs on the market exhibit low tolerability and serious toxicities. They have long treatment durations and difficult administration (iv, im, intralesional).
The innovation encapsulated in this ambitious project is to deliver a new medication for Old World Cutaneous Leishmaniasis (OWCL) patients that is effective, well tolerated and orally administered, as well as being affordable. That could dramatically change the lives of 1 million people around the world
Therefor oral D121 will be evaluated as a new drug to treat Old World CL (OWCL) and the process of clinical development towards registration with a stringent regulatory authority (EMA/FDA) will be accelerated.
Bridging the gap between preclinical and clinical development
D121 is being developed as an immediate-release tablet for the treatment of CL and is currently the most advanced orally formulated drug specifically being developed for this indication. Production (including stability studies), human tolerability (in limited volunteer studies), efficacy in in vitro studies and safety & efficacy studies in animal models all suggest that D121 is a very promising new, safe, orally available, and affordable new candidate for the treatment for cutaneous leishmaniasis (CL).
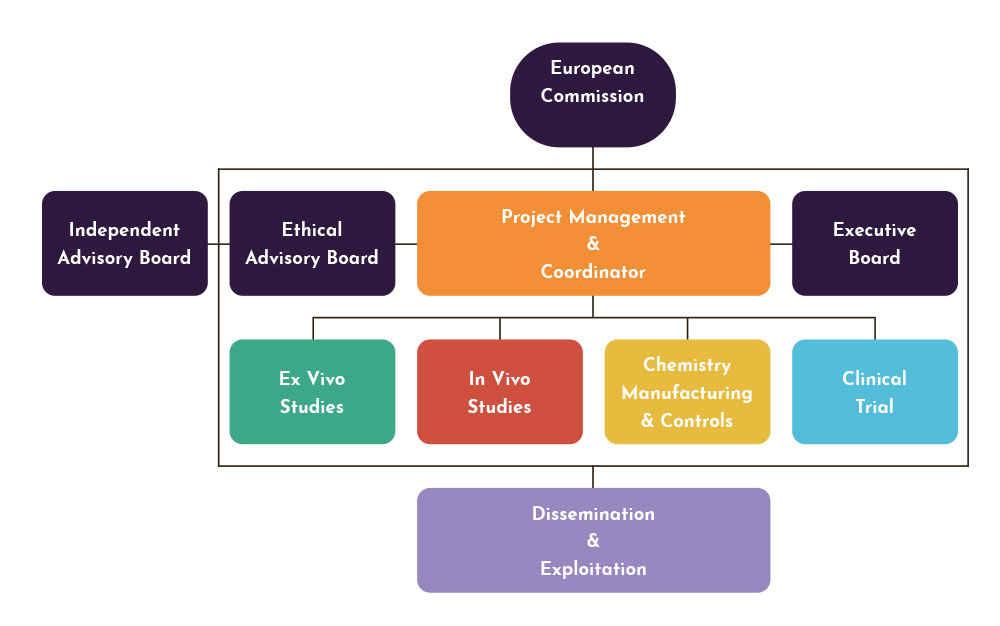
The main concept underpinning this project is to examine critically and to the highest standards the acceptability of D121 as a new potential treatment for OWCL. The classical early clinical development route for a new anti-infective will be followed, by accumulating information that is on the critical path for decisions regarding further developments. This work will involve information obtained by the endemic country partner on the susceptibility of clinical isolates to D121, improvements in production and stability of optmised formulations, detailed PK-PD studies in animal models and Phase 1 studies aimed at dose selection for subsequent (not in this programme) Phase 2 clinical trials.
Specific objectives
The specific objectives are to complete all preclinical investigation and optimisation by:
- refining the manufacture, storage and formulation of D121. See Work package 5 to 7.
- conducting comparative ex vivo efficacy studies in an endemic country (the Islamic Republic of Iran) using fresh CL-causing isolates from clinical studies already in progress, including L.tropica and L. major. See Work package 1 & 2.
- carrying out comparative efficacy studies in animal models of CL, including the determination of skin pharmacokinetic-pharmacodynamic relationships. See Work package 3 & 4.
- conducting pharmacokinetic studies in normal volunteers, investigating the effects of increasing and repeated doses as well as food effects. See Work package 8 to 10.